Hydrogen Fuel Cells- Q&A Part 2
What are the drawbacks to hydrogen fuel cells?
Capacity
The amount of power produced by a fuel cell depends on several factors, including fuel cell type, cell size, temperature at which it operates, and pressure at which the gases are supplied to the cell. A single fuel cell produces less than 1.16 volts—barely enough electricity for even the smallest applications.7 To increase the amount of electricity generated, individual fuel cells are combined in series into a fuel cell “stack.” A typical fuel cell stack may consist
of hundreds of fuel cells.
Cost
Depending on its application, the expenses linked to utilizing a fuel cell can rapidly mount. To ensure power generation, a fixed hydrogen fuel cell system demands the integration of various components apart from the fuel cell itself. Such components comprise storage tanks and/or pipeline systems that receive and convey hydrogen fuel.
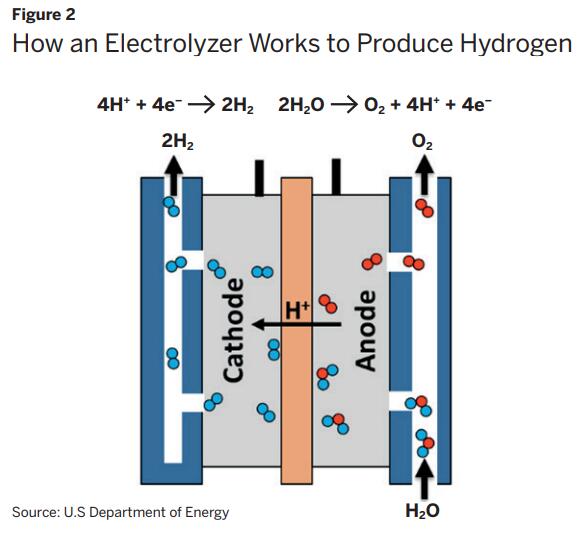
In the event that green hydrogen is produced on-site, supplementary components will be required. These will encompass a renewable energy source like solar panels, plus electrolyzers to create hydrogen, unless adaptable fuel cells are viable. The utilization of flexible fuel cells is increasingly popular and expanding this technology could potentially decrease the expenses linked to utilizing fuel cells for purposes like microgrids that provide backup power.
A fuel cell may be more cost effective in transportation rather than for power generation, as a smaller “stack” is needed to power a vehicle. Fuel cell vehicles need access to hydrogen fueling stations, rather than requiring onsite storage of hydrogen fuel, such as at a home or business facility, where hydrogen storage could raise serious safety concerns.8
Low Efficiency
Because of the multiple steps involved in using a fuel cell to generate electricity, they are much less efficient than batteries. With a hydrogen fuel cell, efficiency loss occurs on multiple levels: the electrolysis process to generate green hydrogen fuel, the costs for transport and storage of the hydrogen, the AC/DC current inversion losses, and the hydrogen to electricity conversion losses. When accounting for these losses, the lifecycle efficiency of a hydrogen fuel cell is 33 percent.9 A battery’s lifecycle efficiency is about 86 percent.
What are potential benefits of hydrogen fuel cells?
Resilience
Despite the disadvantages of fuel cells, they can still serve as a valuable tool for enhancing the resilience of energy-intensive facilities. Provided there is fuel available, a fuel cell has the potential to generate uninterrupted power. This makes it an appropriate backup source for significant energy users like hospitals and data centers, which cannot afford any power interruptions. Hydrogen is an energy-dense fuel that can be retained on-site until needed, or alternatively, produced on-site using renewable energy systems such as solar panels.
No Combustion and Reduced Emissions Potential
When hydrogen is run through a fuel cell, combustion does not occur and the only by-product is water, making fuel cells a cleaner use for hydrogen fuel. When hydrogen is combusted alone or with natural gas, such as in a power plant or for home heating, it produces air pollutants that are harmful to public health.10 It should be noted that if the hydrogen that is used in a fuel cell is generated from natural gas, currently the most common form of hydrogen production, there are still significant emissions impacts upstream. This is due to the increase in methane emissions associated with transporting and storing natural gas, activities that often result in unintended leaks of methane, which is a potent and harmful greenhouse gas.11 Hydrogen produced via electrolysis powered by renewable energy does not have as many emissions concerns, although there is a growing body of research examining the global warming impacts of hydrogen that is released into the atmosphere.12
8. ARUP Department for Business, Energy & Industrial Strategy, “Hy4Heat Safety Assessment Conclusions Report,” static1.squarespace.com, May 2021, https://static1.squarespace.com/static/5b8eae345cfd799896a803f4/t/60e399b094b0d322fb0dadc4/1625528759977/conclusions+inc+QRA.pdf %20 (accessed September 27, 2022).
9. Tsakiris, Aristeidis, “Analysis of Hydrogen Fuel Cell and Battery Efficiency,” c2e2.unepccc.org, March 2019, p. 8, https://c2e2.unepccc.org/wp-content/ uploads/sites/3/2019/09/analysis-of-hydrogen-fuel-cell-and-battery.pdf (accessed September 27, 2022).
10.Cellek, Mehmet Salih and Ali Pınarbaşı, “Investigations on Performance and Emission Characteristics of an Industrial Low Swirl Burner While Burning Natural Gas, Methane, Hydrogen-Enriched Natural Gas and Hydrogen as Fuels,” International Journal of Hydrogen Energy. 43, no. 2 (January 11, 2018): 1194–1207, sciencedirect.com, https://doi.org/10.1016/j.ijhydene.2017.05.107 (accessed September 27, 2022).
11. Howarth, Robert W, “A Bridge to Nowhere: Methane Emissions and the Greenhouse Gas Footprint of Natural Gas,” Energy Science & Engineering 2, no. 2 (June 1, 2014): 47–60, onlinelibrary.wiley.com, https://doi.org/10.1002/ese3.35 (accessed September 27, 2022).
12. Ocko, Ilissa B., and Steven P. Hamburg, “Climate Consequences of Hydrogen Emissions,” Atmospheric Chemistry and Physics 22, no. 14 (July 20, 2022): 9349–68, Acp.copernicus.org, https://doi.org/10.5194/acp-22-9349-2022 (accessed September 27, 2022).
Source: Hydrogen Fuel Cells ANSWERS TO FOUR COMMON QUESTIONS, https://www.cleanegroup.org/wp-content/uploads/Hydrogen-Fuel-Cell-Fact-Sheet.pdf
